In many instances nature has used superhydrophobicity to allow plants and insect to survive under water for long periods of time One example is Salvinia molesta, an extremely invasive fern thatNow up your study game with Learn mode Compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acidsThe hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules The word hydrophobic literally means waterfearing, and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water
Amphipathic Definition And Examples Biology Online Dictionary
What are the 4 types of biomolecules
What are the 4 types of biomolecules-You just studied 29 terms!The biomolecules they are molecules that are generated in living beings The prefixbiomeans life;




Biomolecule Definition Structure Functions Examples Facts Britannica
Work Sheet 2 Molecules of Life/Water 1 List the four major classes of biomolecules found in the human body 2 Draw an ester bond and demonstrate how it is formed from the condensation of two common functional groups 3 Describe the three subclasses of lipids and provide representative examples of each (drawing detailed structures not required)Examples of Hydrophilic Sugar Sugar, or more specifically glucose, is a molecule that many types of cells use as an energy source Enzymes DNA, the information moleculeSome examples of inorganic biomolecules are water, certain gases such as oxygen (O 2) or hydrogen (H 2), NH 3 and NaCl Organic biomolecules Organic biomolecules are based on carbon chemistry These biomolecules are the product of chemical reactions in the body or the metabolism of living beings
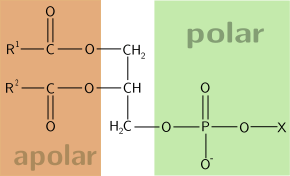
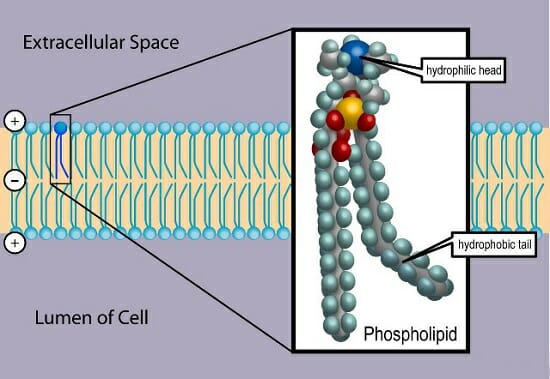
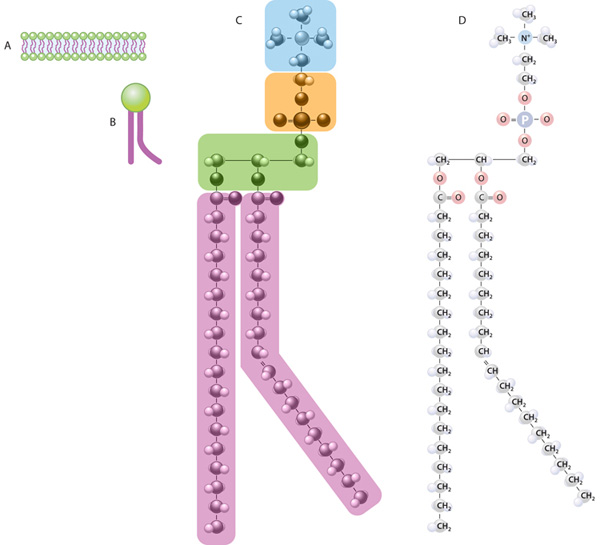
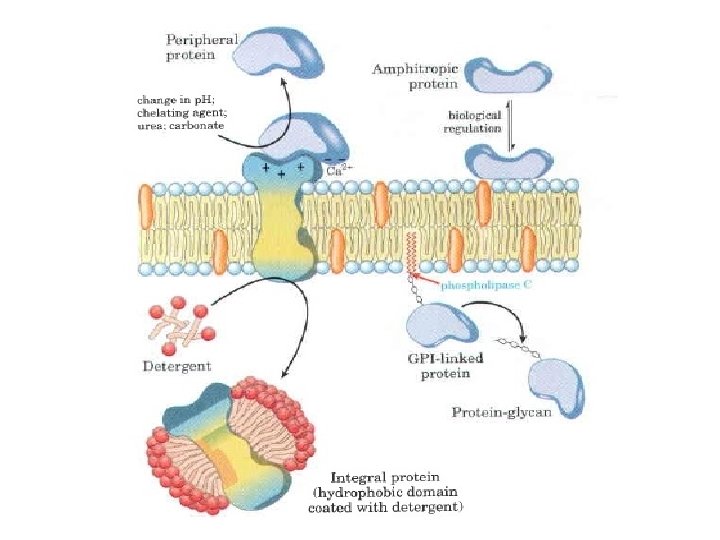
Specifically, there are two fatty acid tails and a phosphate group as the polar head group The phospholipid is an amphipathic molecule, meaning it has a hydrophobic part and a hydrophilic part The fatty acid chains are hydrophobic and cannot interact with water, whereas the phosphatecontaining head group is hydrophilic and interacts with waterIn each of the examples listed above, and in many more examples in nature (Fig1), specialized biomolecules, such as proteins, peptides, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and polysaccharides, have been found or are thought to play a critical role in directing the formation of these hierarchically assembled inorganicAn example of a hydrophobic group is the nonpolar methane molecule Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acid heads that form triglycerides and phospholipids
Taking inspiration from the structure of diatom algae frustules and motivated by the need for new detecting strategies for emerging nanopollutants in water, we analyze the potential of nanoporous silica tablets as metering devices for the concentration of biomolecules or nanoparticles in water The concept relies on the different diffusion behavior that waterAll of them share, in addition, a fundamental relationship between structure and functions, which also involves the environment in which the biomolecule takes place for example, lipids have a hydrophobic side, that is, that repels water , so they are usually organized in the presence of it so that the hydrophilic ends (attracted by water) are in contact with theExamples of carbohydrates are cane sugar, glucose, starch, etc Most of them have a general formula, Cx(H2O)y, and were considered as hydrates of carbon from where the name carbohydrate was derived For example, the molecular formula of glucose (C6H12O6) fits into this general formula, C6(H2O)6 But all the compounds which fit into this formula



Phospholipid Definition Structure Function Biology Dictionary



1
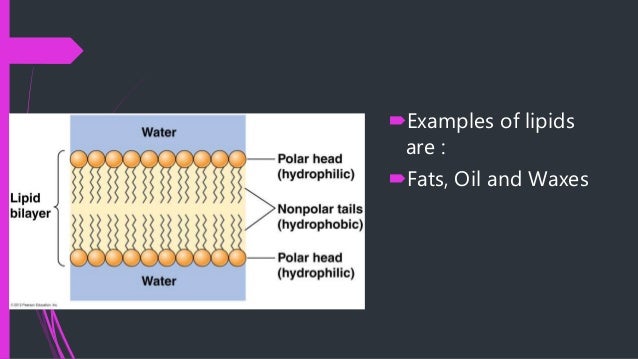
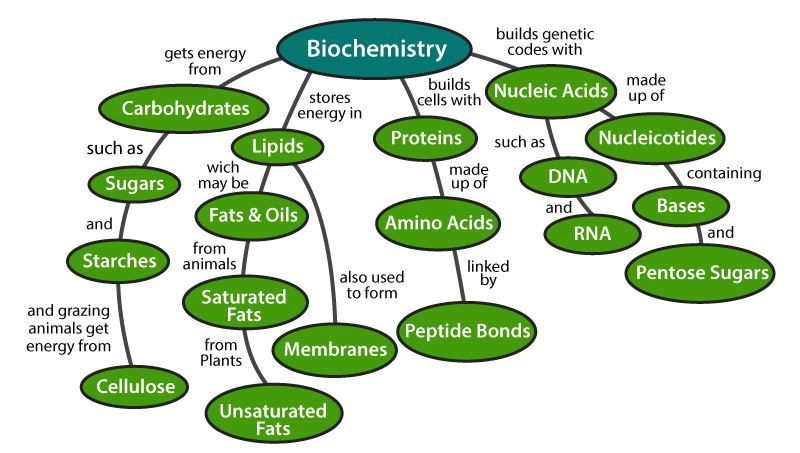
Since many biomolecules are either polar or charged, water readily dissolves these hydrophilic compounds Water is a poor solvent, however, for hydrophobic molecules such as lipids Nonpolar molecules experience hydrophobic interactions in water the water changes its hydrogen bonding patterns around the hydrophobic molecules to produce a cageBiological membranes are mainly composed of two biomolecules, lipids and proteins The spatial conformation of lipids is in the form of a bilayer, with the hydrophobic tails pointing inwards, and the hydrophilic heads pointing outwards The membrane is a dynamic entity and its components experience frequent movements ReferencesBiomolecules There are four basic kinds of biological macromolecules carbohydrates, lipids, proteins, and nucleic acids These polymers are composed of different monomers and serve different functions Carbohydrates molecules composed of sugar monomers They are necessary for energy storage




Biomolecule Definition Structure Functions Examples Facts Britannica



The Biological Building Blocks Cancerquest
View Unit 2 LTs Biomolecules AP Biopdf from BIOLOGY 1 at St Norbert College 1 AP Biology Unit 2 Learning Targets Biomolecules 1 What is the essential element that forms the "backbone"All biomolecules share in common a fundamental relationship between structure and function, which is influenced by factors such as the environment in which a given biomolecule occurs Lipids, for example, are hydrophobic ("waterfearing");Type of hydrophilic head present Glycolipids are lipids whose head contains oligosaccharides with 115 saccharide residues Phospholipids contain a positively charged head which are linked to the negatively charged phosphate groups Sterols, whose head contain a steroid ring Example steroid



The Different Biomolecules




Biological Molecules Biology I
What are the 4 types of biomolecules?The remarkable progress in the field of ionic liquids (ILs) in the last two decades has involved investigations on different aspects of ILs in various conditions The nontoxic and biocompatible nature of ILs makes them a suitable substance for the storage and application of biomolecules In this regard, the aqueous IL solutions have attracted a largeThe strategy of combining biomolecules with polymer membranes changes depending on the nature of the biomolecules in terms of their hydrophobic or hydrophilic characteristics To date, the majority of biomolecules have been combined with polymeric membrane by either attaching them to the hydrophilic part membrane surface or by inserting them



Biomolecules In Living Organisms The Four Types Of Biomolecules




Hydrophilic Definition Interaction Video Lesson Transcript Study Com
Nanoparticle–biomolecule interactions can be quite unlike those of bulk material of the same composition because the nanoparticle's effective surface charge, stability, solubility, and hydration are greatly affected by the local milieu's pH, ionic strength, temperature, and organic/molecular composition (eg, proteins, lipidsIn water, many spontaneously arrange themselves in such a way that the hydrophobic ends of the molecules are protected from the water, while the hydrophilicPhosphate group Hydrophilic molecules, are those which has the tendency to dissolve readily in water DNA is a hydrophilic in nature because of phosphorous present in phosphodiester bonds which has negative charge due to surplus electron




Role Of The Hydrophobic And Hydrophilic Sites In The Dynamic Crossover Of The Protein Hydration Water Sciencedirect



Protein Tertiary Structure Wikipedia
Fat is an essential part of your diet It provides energy, absorbs certain nutrients and maintains your core body temperature The main function of a lipid, or in other words fat, is to help protect organs from getting damaged, helps store energy when youPhospholipids are lipids containing phosphate groups eg phosphoglyceride They have a hydrophilic polar head and a hydrophobic nonpolar tail Question 9Wearable materials are endowed with synthetic biology circuits to detect biomolecules, including SARSCoV2 RNA towards enclosed hydrophilic paper networks use nature of the sensors and




Amphipathic Definition And Examples Biology Online Dictionary



Cell Membranes Learn Science At Scitable
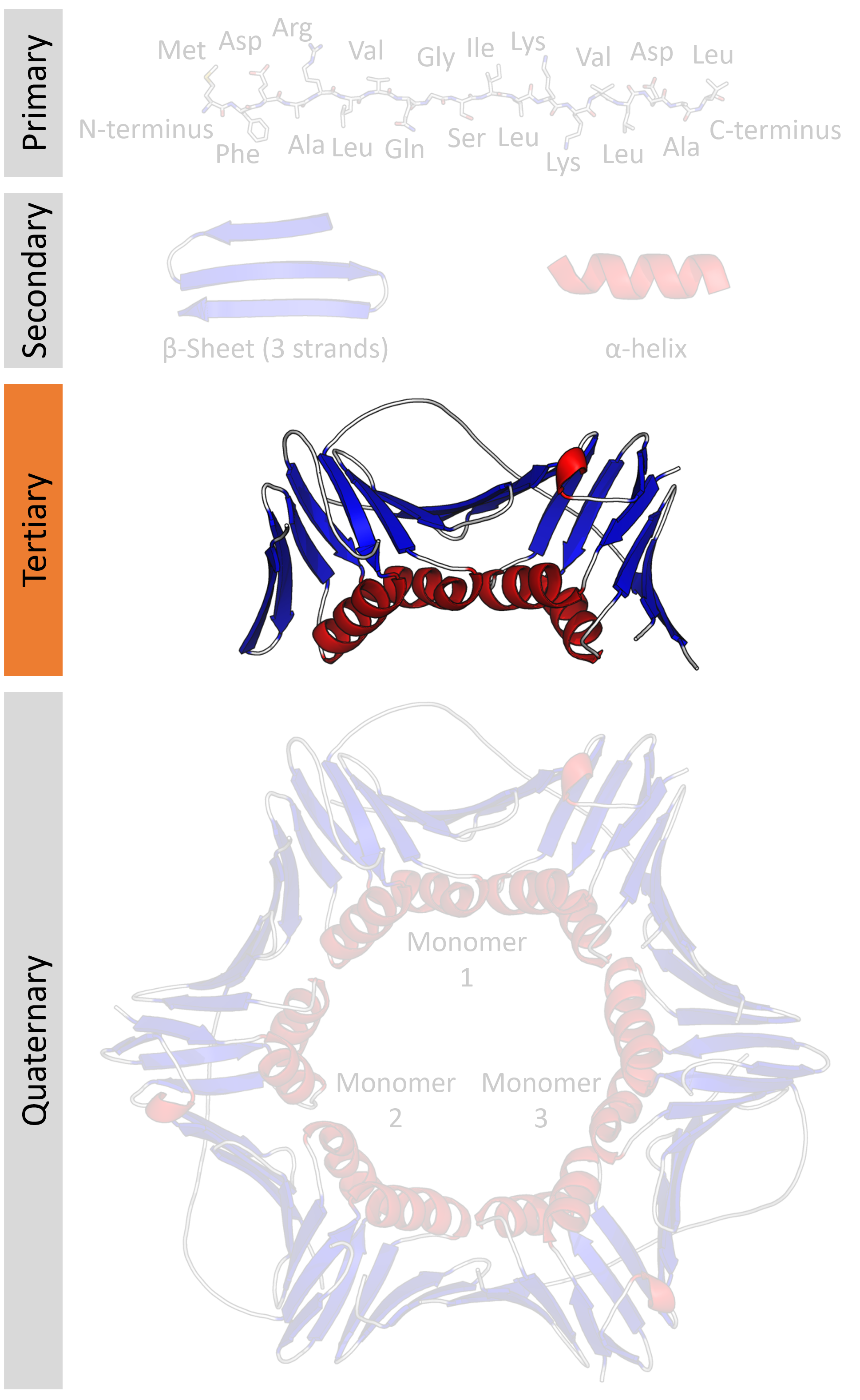
Physically enzymes behave as colloids or as substances of high molecular mass They are hydrophilic in nature They are amphoteric in nature Thus, behave as alkali with acid and as acid with alkali (iv) Reversible in action Enzymes can accelerate the reaction in either directions eg, Sucrase enzyme hydrolyse sucrose into glucose and fructoseD Explain the chemical nature, structure and role of phospholipids in biological membrane Answer Phospholipids consist of a hydrophilic (or 'water loving') head and a hydrophobic (or 'water fearing') tail Phospholipids like to line up and arrange themselves into two parallel layers, called a phospholipid bilayerStructure and function of Biomolecules 8 STRUCTURE AND FUNCTION OF BIOMOLECULES Table of contents 1 Introduction 9 2 Proteins 13 o The Amino Acids o The Peptide bond o The Protein Conformation o The secondary structures αhelix and βsheet 3 Lipids 25 o Fatty Acids o Hormones derived from Fatty Acids



1
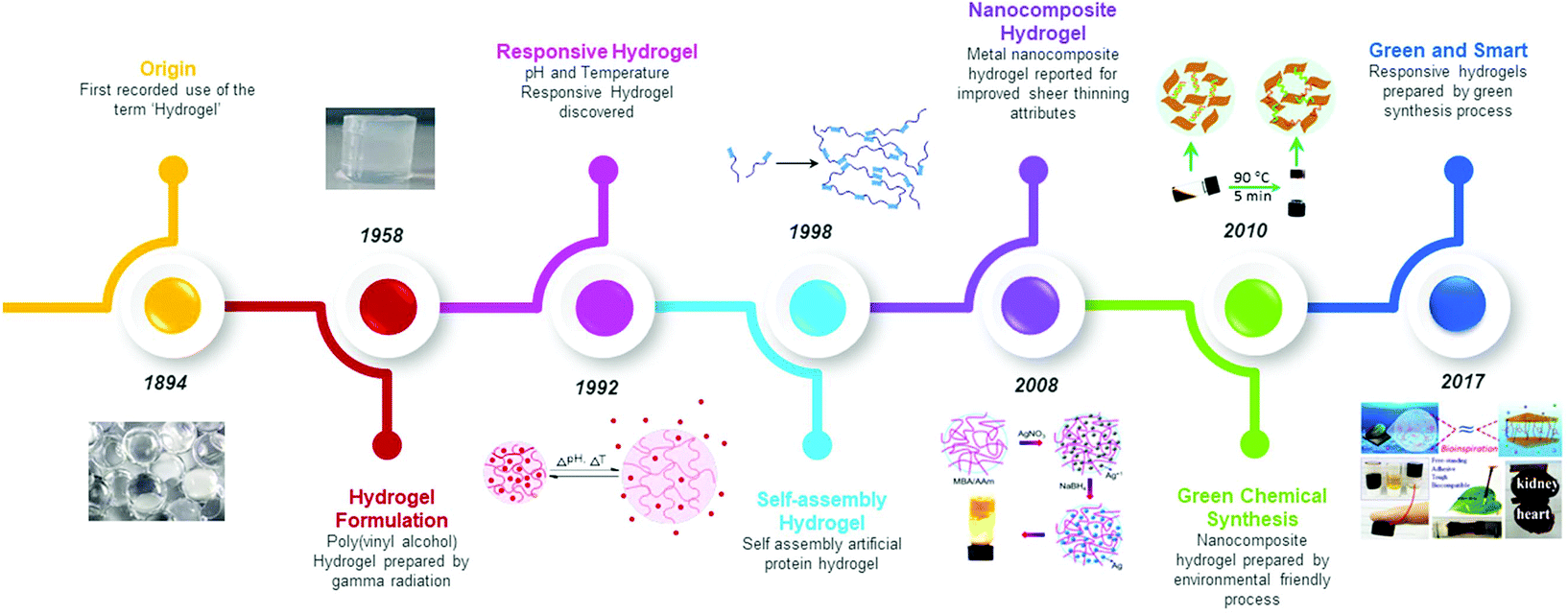



Biomolecule Assisted Synthesis Of Biomimetic Nanocomposite Hydrogel For Hemostatic And Wound Healing Applications Green Chemistry Rsc Publishing Doi 10 1039 D0gcd
Monomers (or building blocks) of carbohydrates are ____ Nice work!Introduction The αcarbon of an amino acid is chiral, so each one has L and D isomersAll protein amino acids, however, only exist in their L forms While the figure to the left depicts the correct functional groups, amino acids are conventionally written in their zwitterionic forms, where the amine group is protonated and the carboxyl group is deprotonated due to the pH level of mostAnd the phosphate backbone is exposed to surface, where they can interact easily with water




Hydrophobic Definition And Examples Biology Online Dictionary




What Are Some Examples Of Hydrophilic Substances Quora
Because the natural world is full of hydrophobic and hydrophilic surfaces, the basics of the phenomenon have been known by scientists for at least two centuries For example, the lotus leaf is a wellknown example of a hydrophobic material, protecting the waterdwelling plant from becoming waterloggedAn example of these amphiphilic molecules is the lipids that comprise the cell membrane Another example is soap, which has a hydrophilic head and a hydrophobic tail, allowing it to dissolve in both water and oil Hydrophilic and hydrophobic molecules are also known as polar molecules and nonpolar molecules, respectively Some hydrophilicThere are various methods for coupling biomolecules with sensors including entrapment into membranes, physical adsorption, entrapment into a matrix, or covalent bonding 42, 44 The high water content and hydrophilic nature of hydrogels are similar to the voidfilling component of the extracellular matrix and render them intrinsically




What Is Hydrophilic In Biology And What Are Some Examples Quora
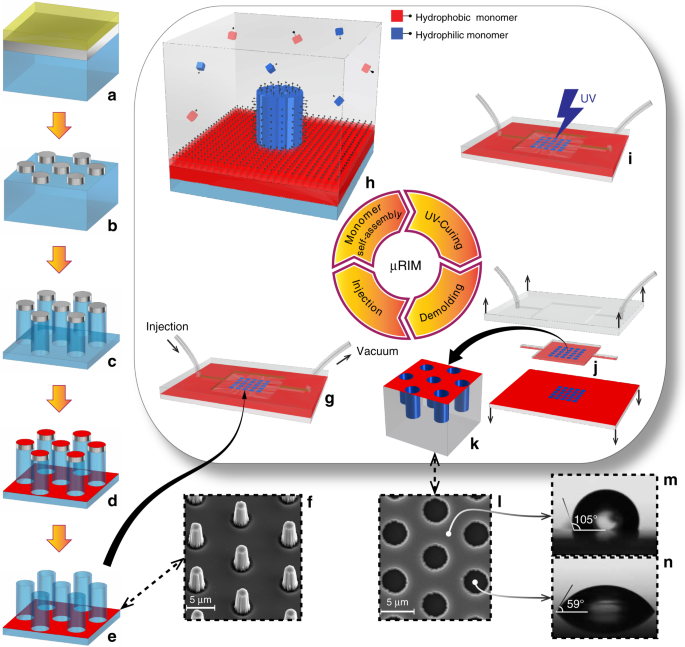



Reaction Injection Molding Of Hydrophilic In Hydrophobic Femtolitre Well Arrays Microsystems Nanoengineering
Therefore, a biomolecule is a molecule produced by a living being Living beings are made up of different types of molecules that carry out various functions necessary for life In nature, there are biotic (living) and abiotic (nonlivingUp to10%cash backMolecular dynamics simulations have been used to investigate the confinement packing characteristics of small hydrophilic (Nacetylglycinemethylamide, Nagma) and hydrophobic (Nacetylleucinemethylamide, Nalma) biomolecules in large diameter singlewall carbon nanotubes (SWCNTs) We find that hydrophilic biomoleculesBiomolecules 1 Biomolecules draarif 2 Chemicals or molecules present in the living organisms are knownas BiomoleculesThe sum total of different types of biomolecules, compounds and ions present in a cell is called as cellular poolBiomolecules are compounds of carbonHence the chemistry of living organisms is organized around carbonCarbon is the most




Hydrophilic Definition Interaction Video Lesson Transcript Study Com




Hydrophobic Definition And Examples Biology Online Dictionary
High molecular weight biomolecules are becoming more and more important in the development of new therapeutic drugs However, the hydrophilic nature of such molecules creates a major limitation for their applicationpoor penetration through biological membranes In 1994, a new class of peptidescellpenetrating peptides (CPPs)was discoveredFor example, the hydrophilic peptide sequence Aβ(11–17) generally does not form characteristic amyloid fibrils owing to the absence of sufficient hydrophobic sequencesBecause of the hydrophilic nature of CNCs the first attempts to prepare thermosetting nanocomposites were done with hydrophilic resins For example, Nakagaito and Yano (04) used a phenolformaldehyde matrix to produce high strength nanocomposites



Carbohydrates Are The Most Abundant Biomolecules On Earth




Biological Molecules Biology I
On the other hand in the right hand side you can see these structures for example vitamin b9 is a folic acid this has a functional group acid group is acid group all hetero atoms so many hetero atoms are there which are hydrophilic in nature So, this overall this molecule has a lot of charge separation it is a polar molecule and a hydrophilicThe hydrophilic character of a molecule can be described as its hydrophilicity Hydrophilic molecules are polar molecules Water molecules are polar molecules, which allows polar molecules to get dissolved in water These hydrophilic molecules are called hydrophiles• Macroscopic examples tendons, ligaments, hair, nails (collagen, keratin) • Cellular level examples Cellular level examples actin actin,, tubulintubulin Protein Functions • Transport • Proteins that carry other molecules from one place to another • Examples hemoglobin, Examples hemoglobin, kinesins kinesins Protein Functions • Catalysis
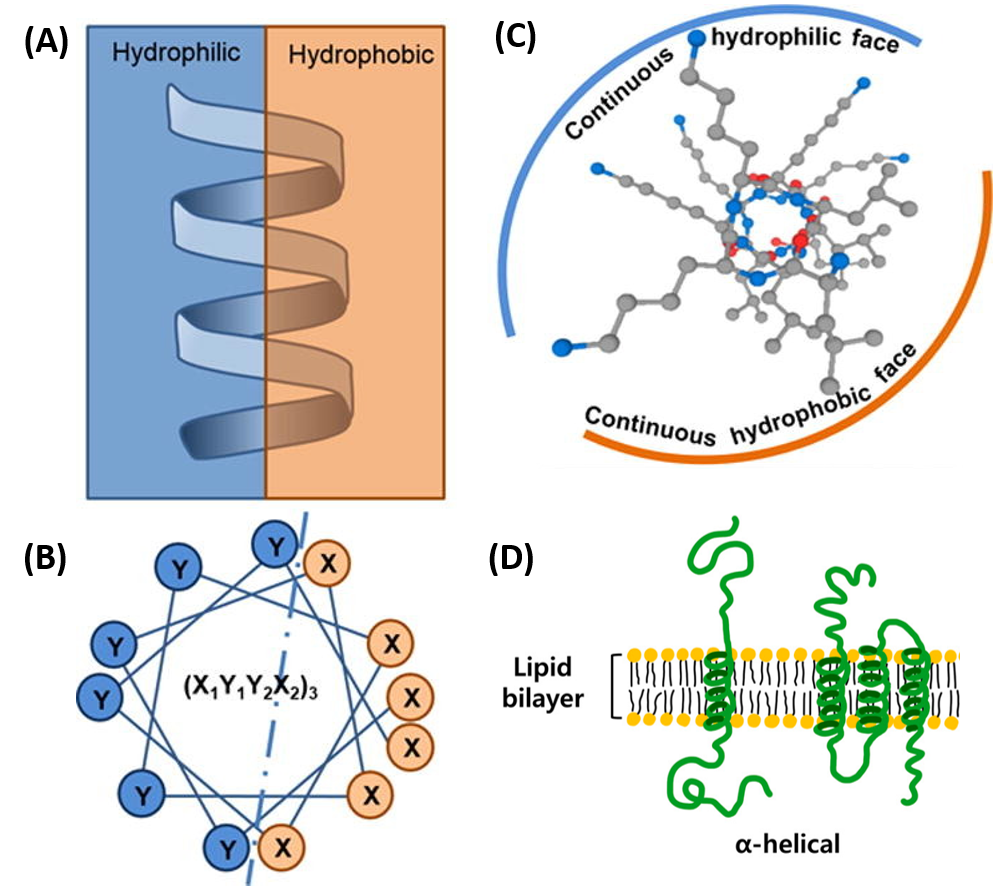



Chapter 2 Protein Structure Chemistry



1




Biological Molecules Biological Principles



Biomolecules 1 Describe The Difference Between A Monomer




Hydrophobic Interactions Chemistry Libretexts
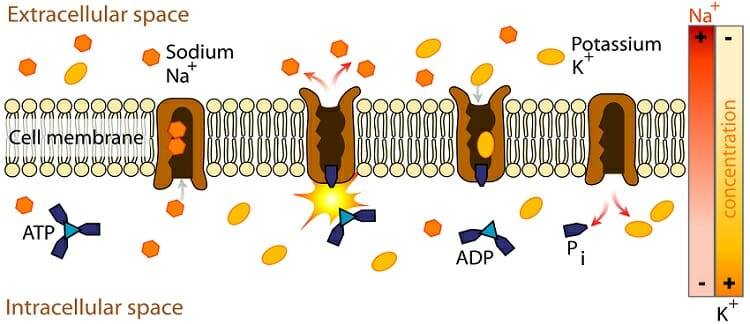



Hydrophilic Definition And Examples Biology Dictionary




Combinational Effect Of Cell Adhesion Biomolecules And Their Immobilized Polymer Property To Enhance Cell Selective Adhesion



1
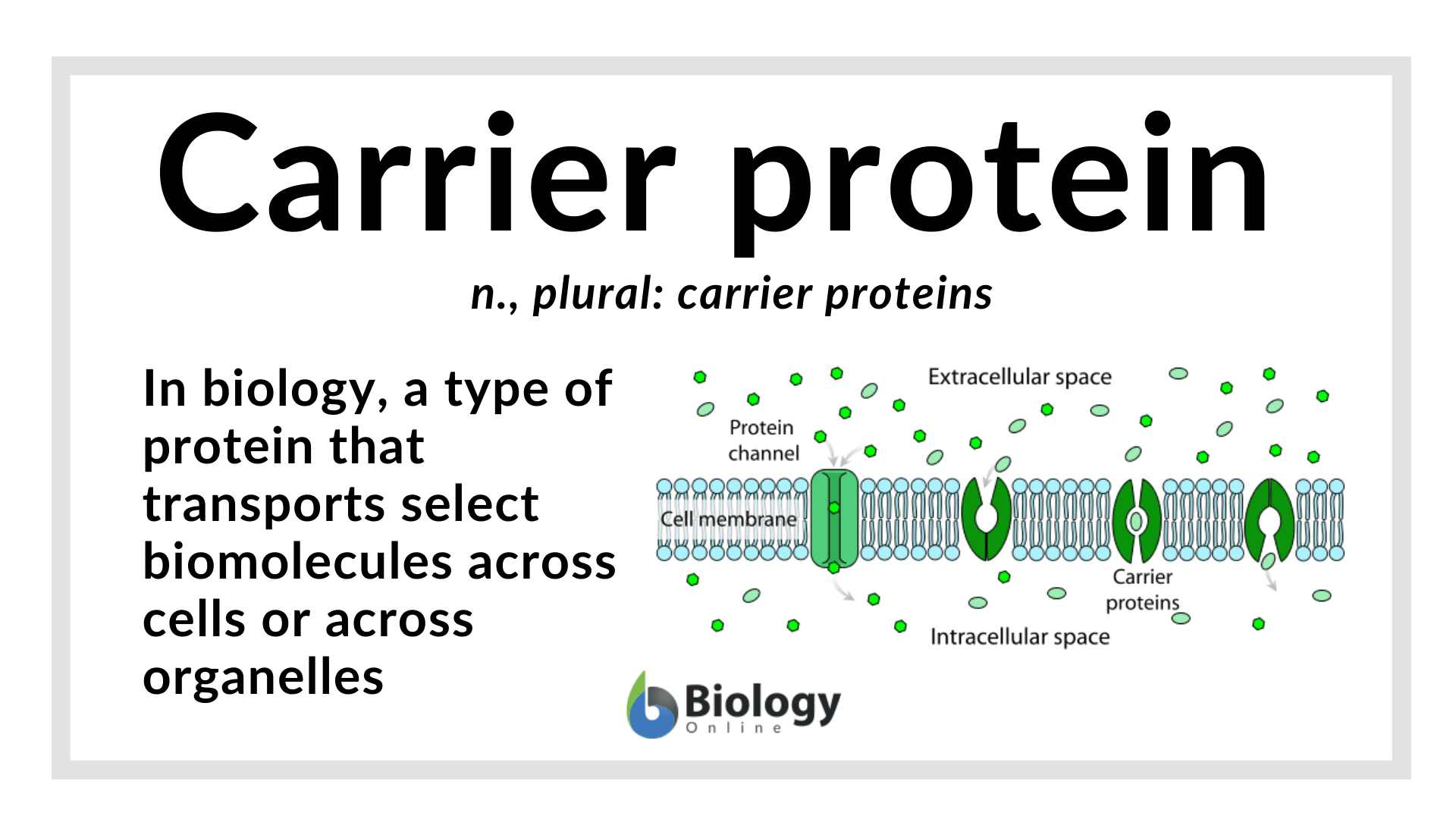



Carrier Protein Definition And Examples Biology Online Dictionary




What Are Biomolecules And Why Are They So Important



2 3 Biological Molecules Concepts Of Biology 1st Canadian Edition
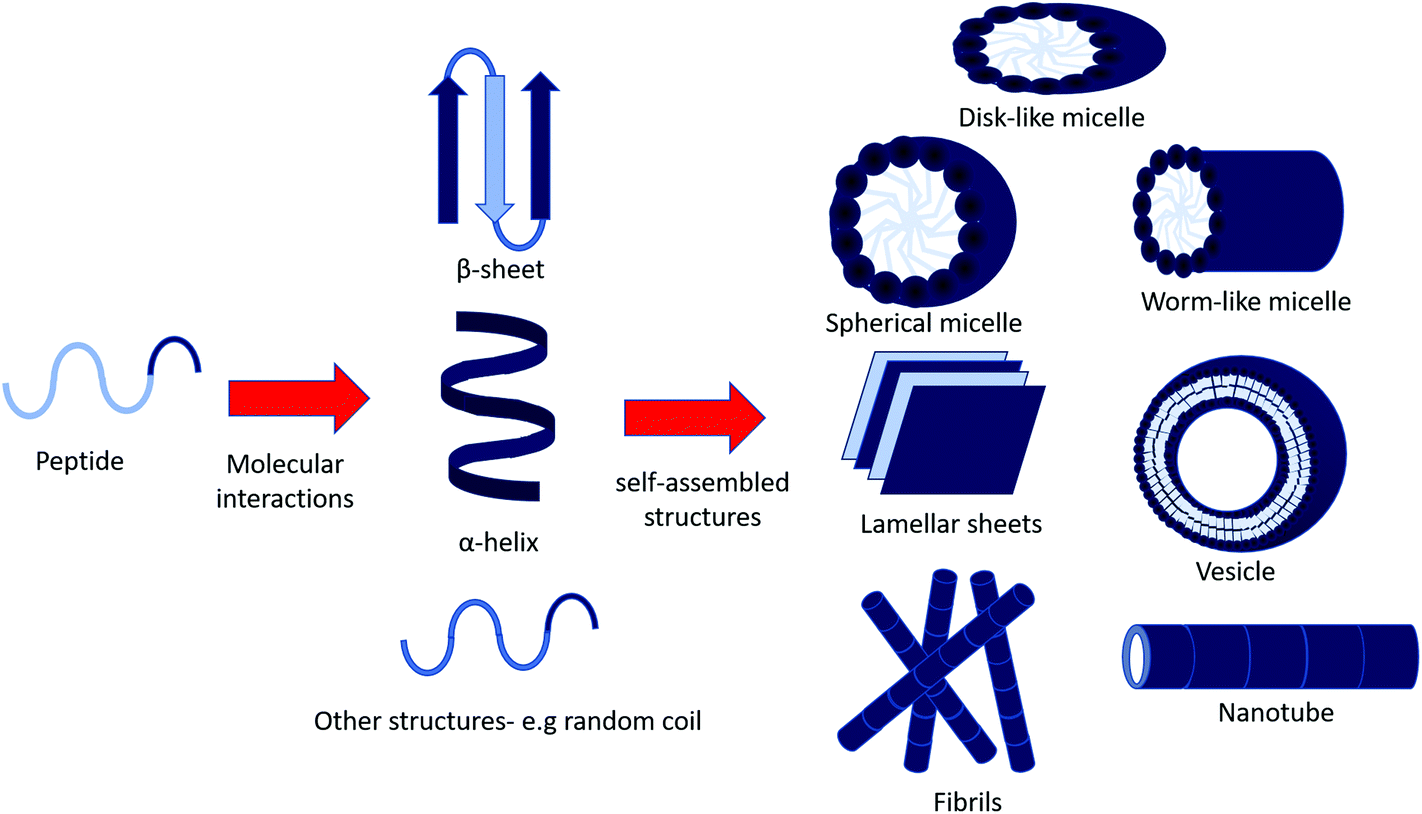



Self Assembly Of Bioactive Peptides Peptide Conjugates And Peptide Mimetic Materials Organic Biomolecular Chemistry Rsc Publishing




Biological Molecules Biology I



Wobble Trna Modification And Hydrophilic Amino Acid Patterns Dictate Protein Fate Nature Communications




Biomolecule Definition Structure Functions Examples Facts Britannica




Quirky Facts About Biomolecules You Probably Didn T Know




Hydrophilic Definition And Examples Biology Online Dictionary
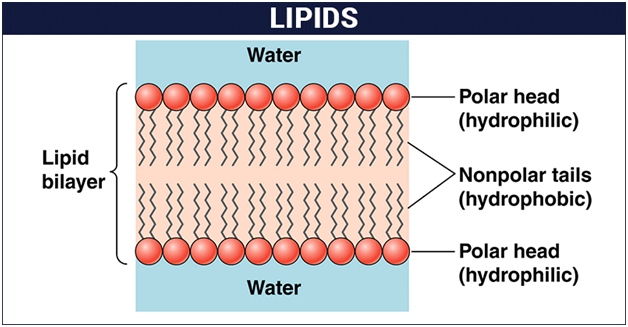



What Are Lipids Definition Structure Classification Of Lipids



Quirky Facts About Biomolecules You Probably Didn T Know



Biomolecules




Organic Compounds Boundless Anatomy And Physiology
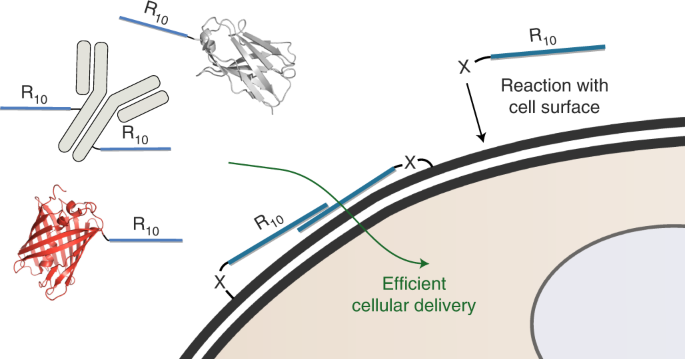



Cellular Uptake Of Large Biomolecules Enabled By Cell Surface Reactive Cell Penetrating Peptide Additives Nature Chemistry




Properties Of Hydrophilic Biomolecules Used In This Study Download Table



Biomolecules
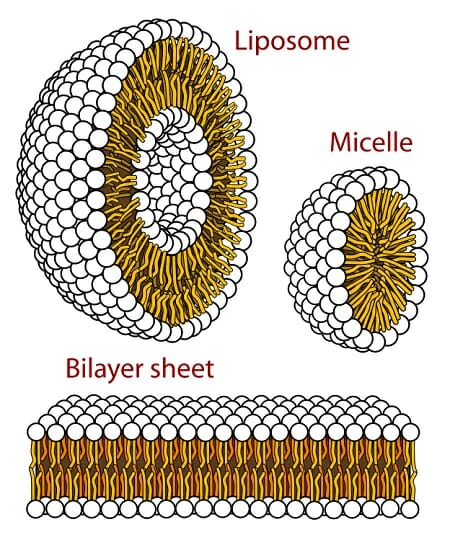



Hydrophobic Definition And Examples Biology Dictionary




Biomolecule Definition Structure Functions Examples Facts Britannica




Biological Molecules You Are What You Eat Video Khan Academy




Structure Of Major Plant Biomolecules With Example Chemicals Download Scientific Diagram




Quirky Facts About Biomolecules You Probably Didn T Know




Polysaccharides Properties Functions And Applications Conduct Science
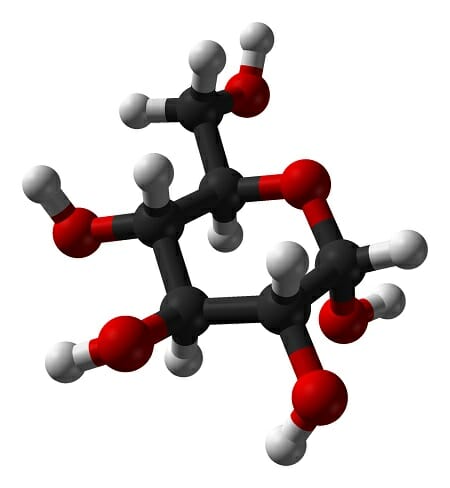



Hydrophilic Definition And Examples Biology Dictionary




Amino Acids Structure Classification Properties With Videos Examples




Biomacromolecules Meaning Types And Videos With Solved Examples




Biomolecule Definition Structure Functions Examples Facts Britannica
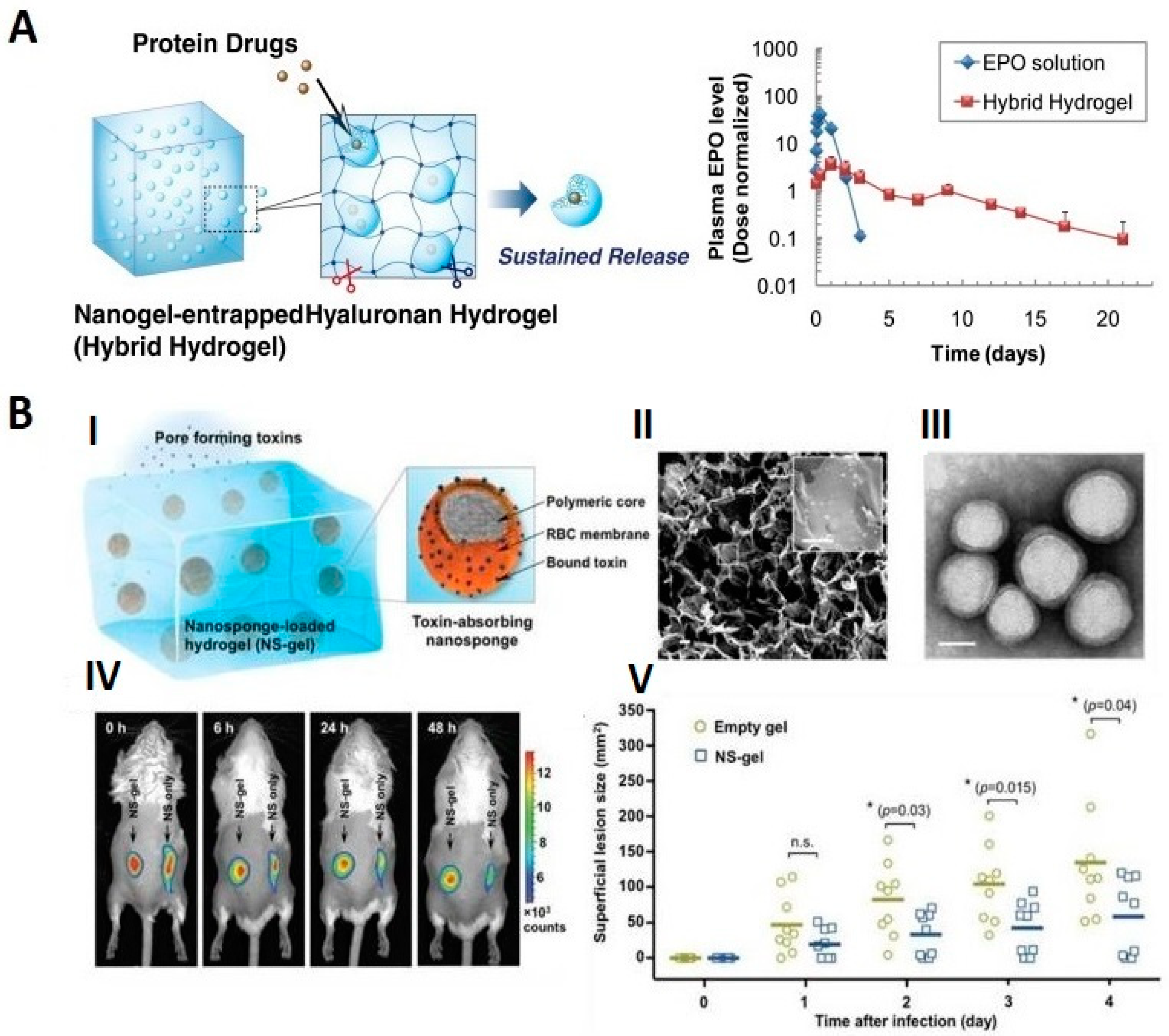



Molecules Free Full Text Recent Advances In Degradable Hybrids Of Biomolecules And Ngs For Targeted Delivery Html



Solved 3 Name The Four Types Of Small Biological Molecules Which Three Are Capable Of Forming Polymeric Structures What Are The Names Of The Pol Course Hero




Folates Stability And Interaction With Biological Molecules Sciencedirect




Amino Acids Structure Classification Properties With Videos Examples




Hydrophilic Definition And Examples Biology Online Dictionary




Hydrophilic Definition And Examples Biology Online Dictionary




Frustration In Biomolecules Abstract Europe Pmc




Forces Involved In Biomolecules Immobilization Onto Natural Fibers A Download Scientific Diagram




Water Follows Polar And Nonpolar Protein Surface Domains Pnas




Bio 111 Biomolecules And Cells Biomolecules Cell Biology




Explained Hydrophobic And Hydrophilic Mit News Massachusetts Institute Of Technology
:max_bytes(150000):strip_icc()/amphipathic-assemblies-bf23d9a24ce94738a272b0b4633a2b0e.jpg)



What Are Amphipathic Molecules Definition Examples




Hydrophile Wikipedia
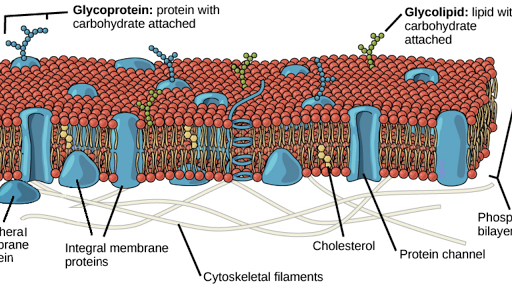



Structure Of The Plasma Membrane Article Khan Academy
/antibody-1igt-562598423-58c861f35f9b58af5c557e0d.jpg)



Glycoprotein Definition And Function




Biomolecules Free Full Text Structure Of The Hydrophobic Core Determines The 3d Protein Structure Verification By Single Mutation Proteins Html




Lipid Definition Structure Examples Functions Types Facts Britannica
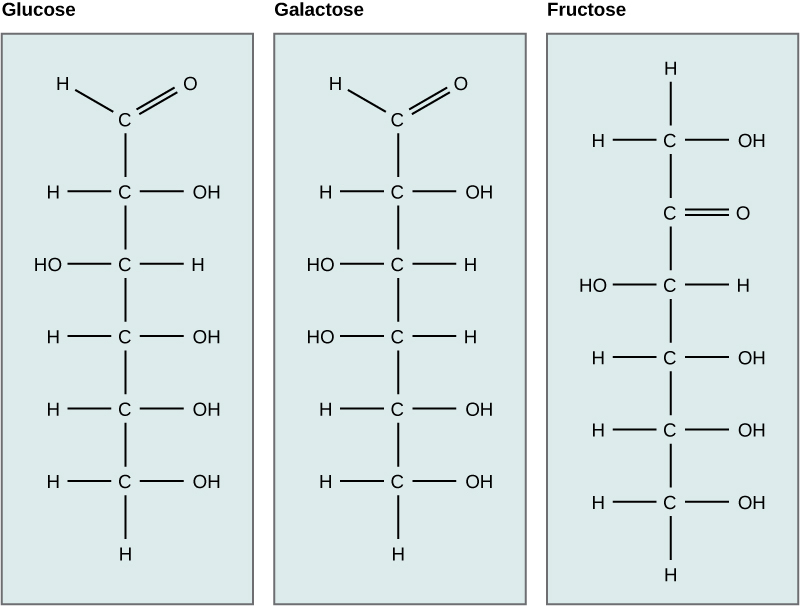



2 3 Biological Molecules Biology Libretexts




Classification Of Amino Acids Video Khan Academy
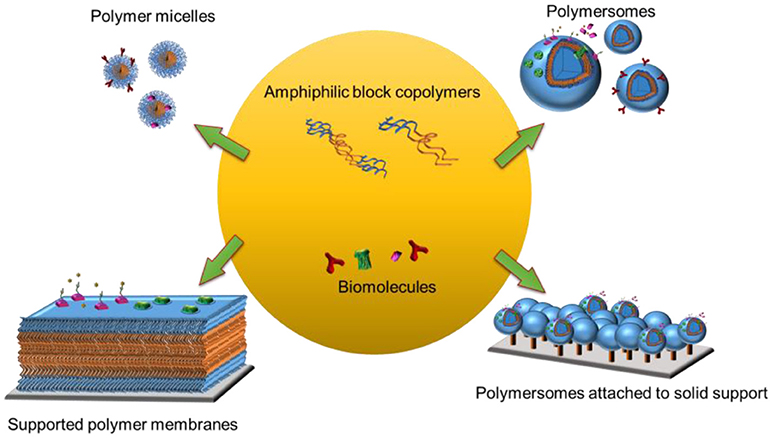



Frontiers Biomolecules Turn Self Assembling Amphiphilic Block Co Polymer Platforms Into Biomimetic Interfaces Chemistry




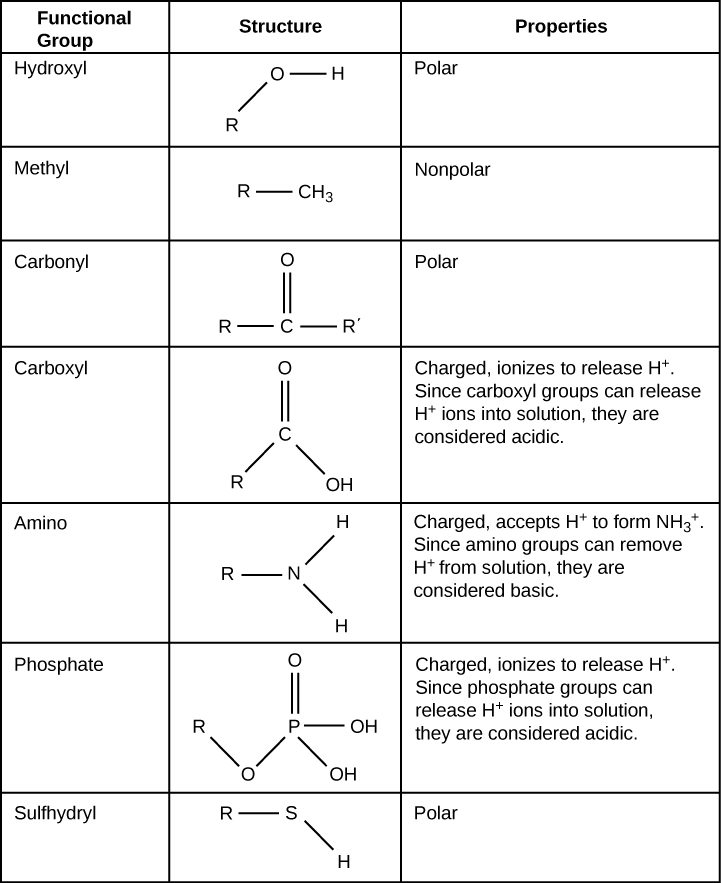
1 9 Functional Groups Biology Libretexts




Biomolecule Wikipedia
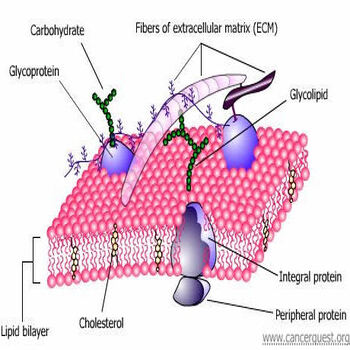



The Biological Building Blocks Cancerquest
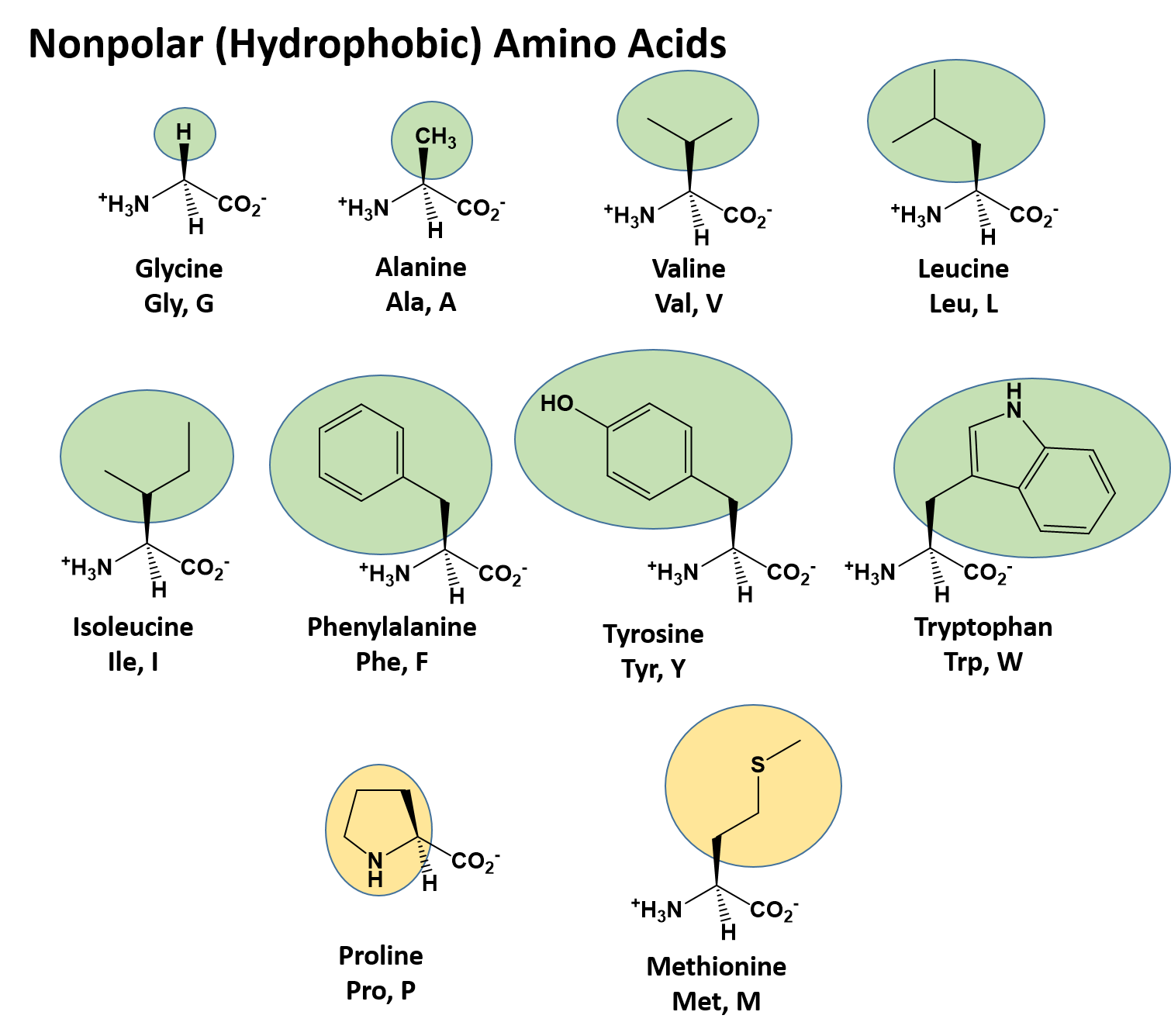



Ch103 Chapter 8 The Major Macromolecules Chemistry
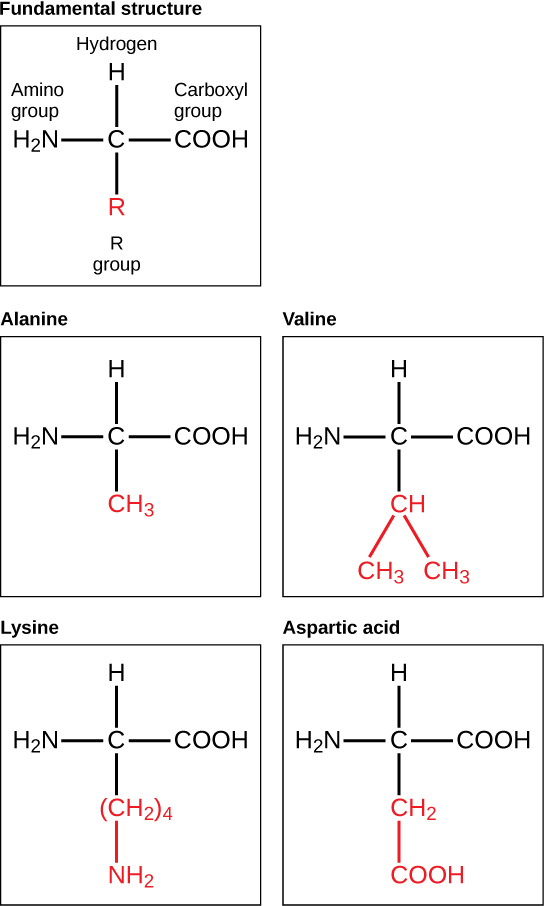



4 1 Biological Molecules Human Biology



Brain Brooder




Properties Of Hydrophilic Biomolecules Used In This Study Download Table



The Biological Building Blocks Cancerquest




Biomolecules And Polymers



Hydrophobic Modifications Of Biomolecules An Introduction Springerlink
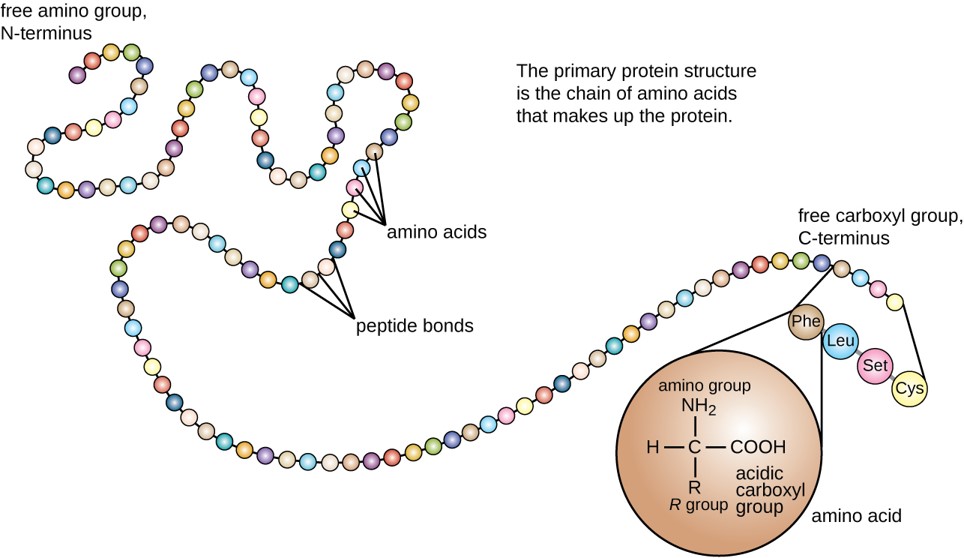



Ch103 Chapter 8 The Major Macromolecules Chemistry
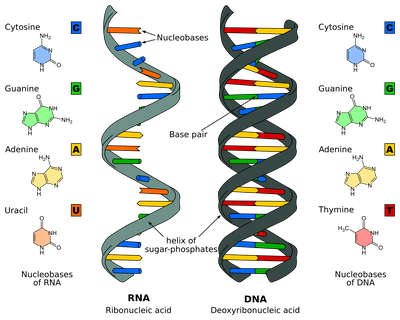



Quirky Facts About Biomolecules You Probably Didn T Know




Biomolecule Definition Structure Functions Examples Facts Britannica
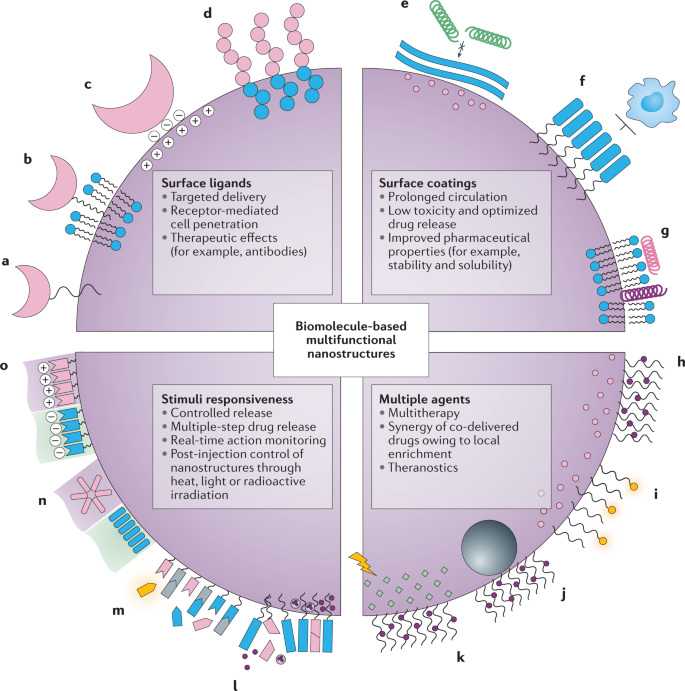



Multifunctional Biomolecule Nanostructures For Cancer Therapy Nature Reviews Materials




Biomolecule Definition Structure Functions Examples Facts Britannica




Hydrophilic Definition And Examples Biology Online Dictionary
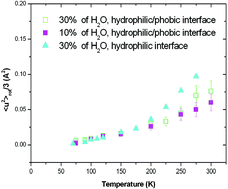



Water Hydrogen Bond Analysis On Hydrophilic And Hydrophobic Biomolecule Sites Physical Chemistry Chemical Physics Rsc Publishing




Multiproduct Microalgae Biorefineries Mediated By Ionic Liquids Trends In Biotechnology




Unit 2 Biomolecules Notes 4 Intro To Biomolecules Lipids Ppt Download
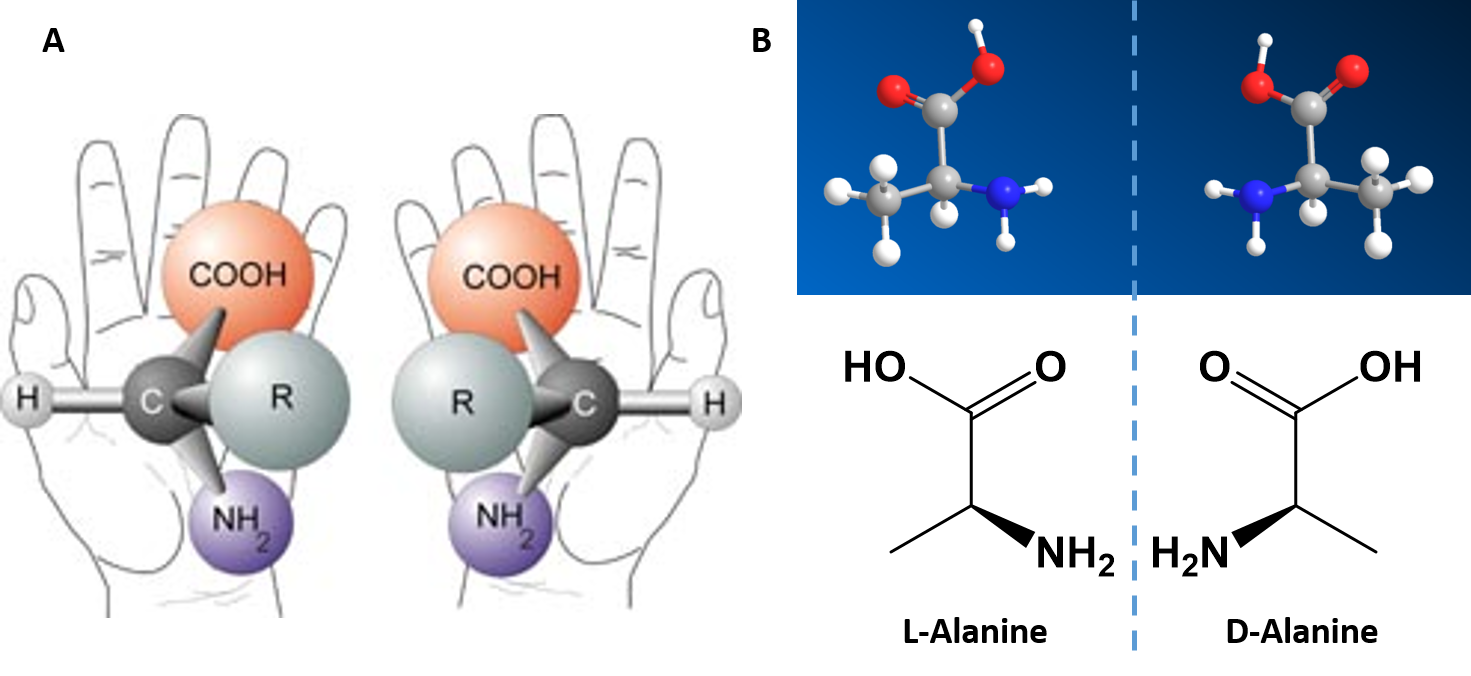



Chapter 2 Protein Structure Chemistry
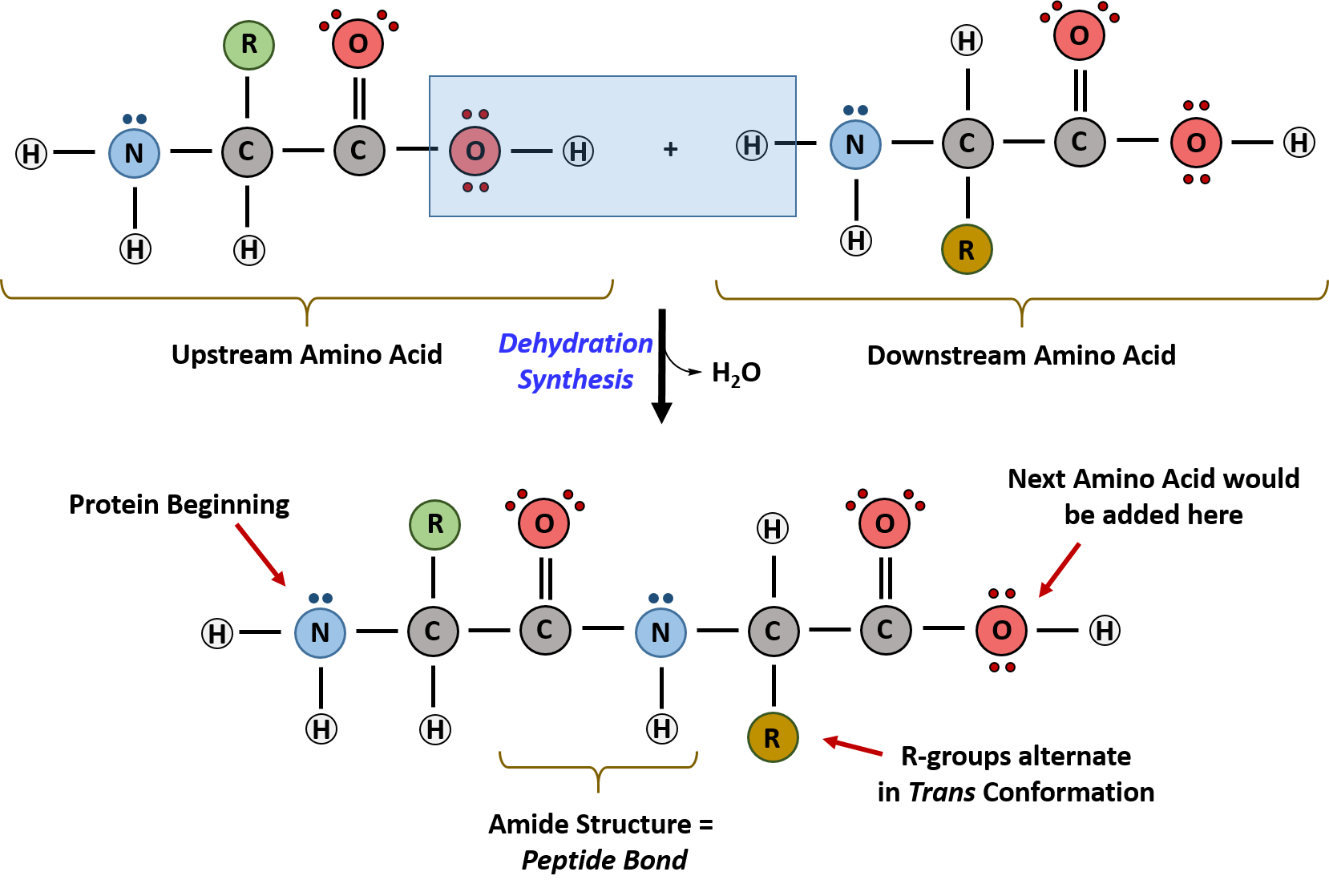



Ch103 Chapter 8 The Major Macromolecules Chemistry
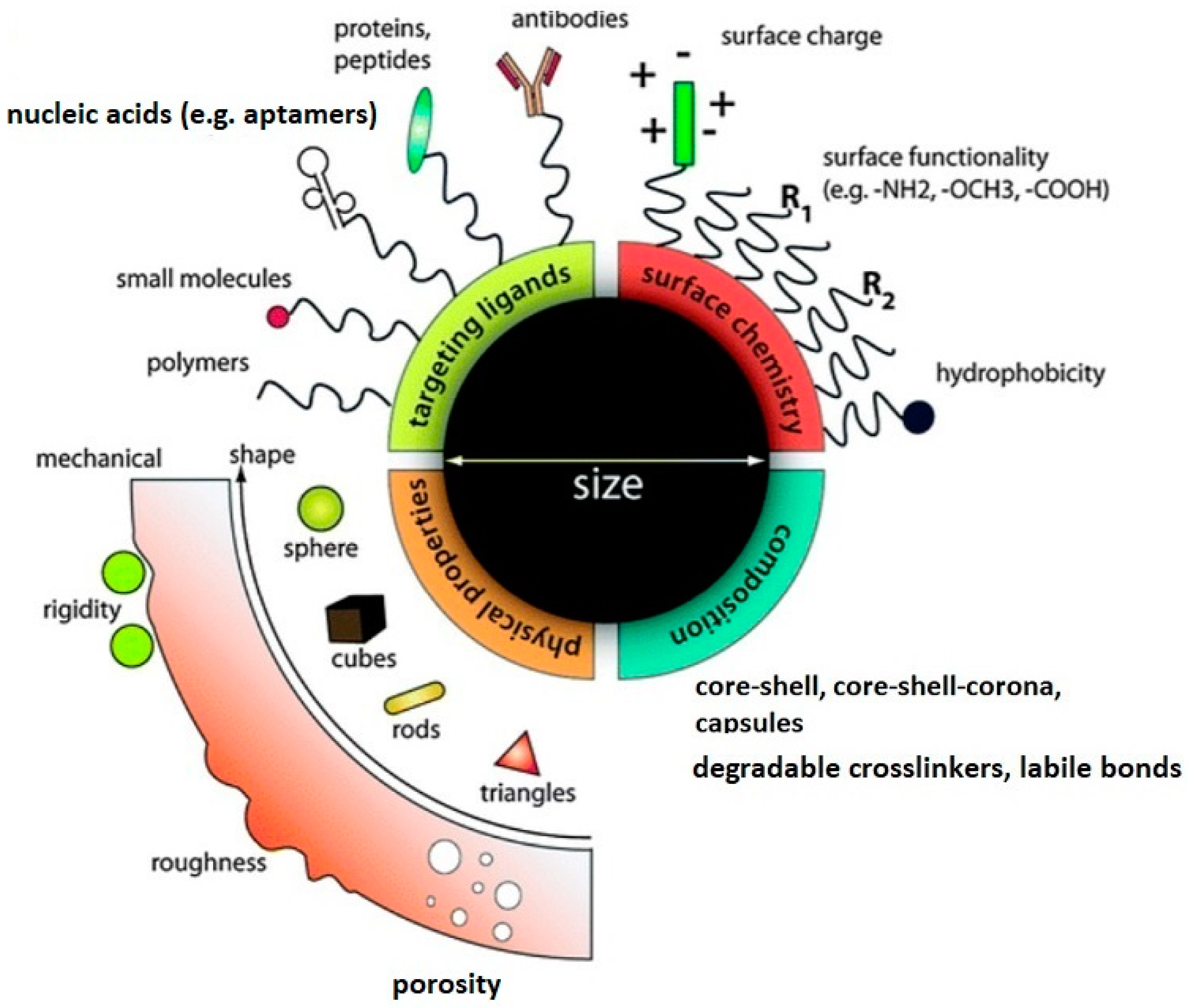



Molecules Free Full Text Recent Advances In Degradable Hybrids Of Biomolecules And Ngs For Targeted Delivery Html




Classes Of Organic Compounds Boundless Chemistry




Biological Molecules Biology I



What Is Hydrophilic In Biology And What Are Some Examples Quora
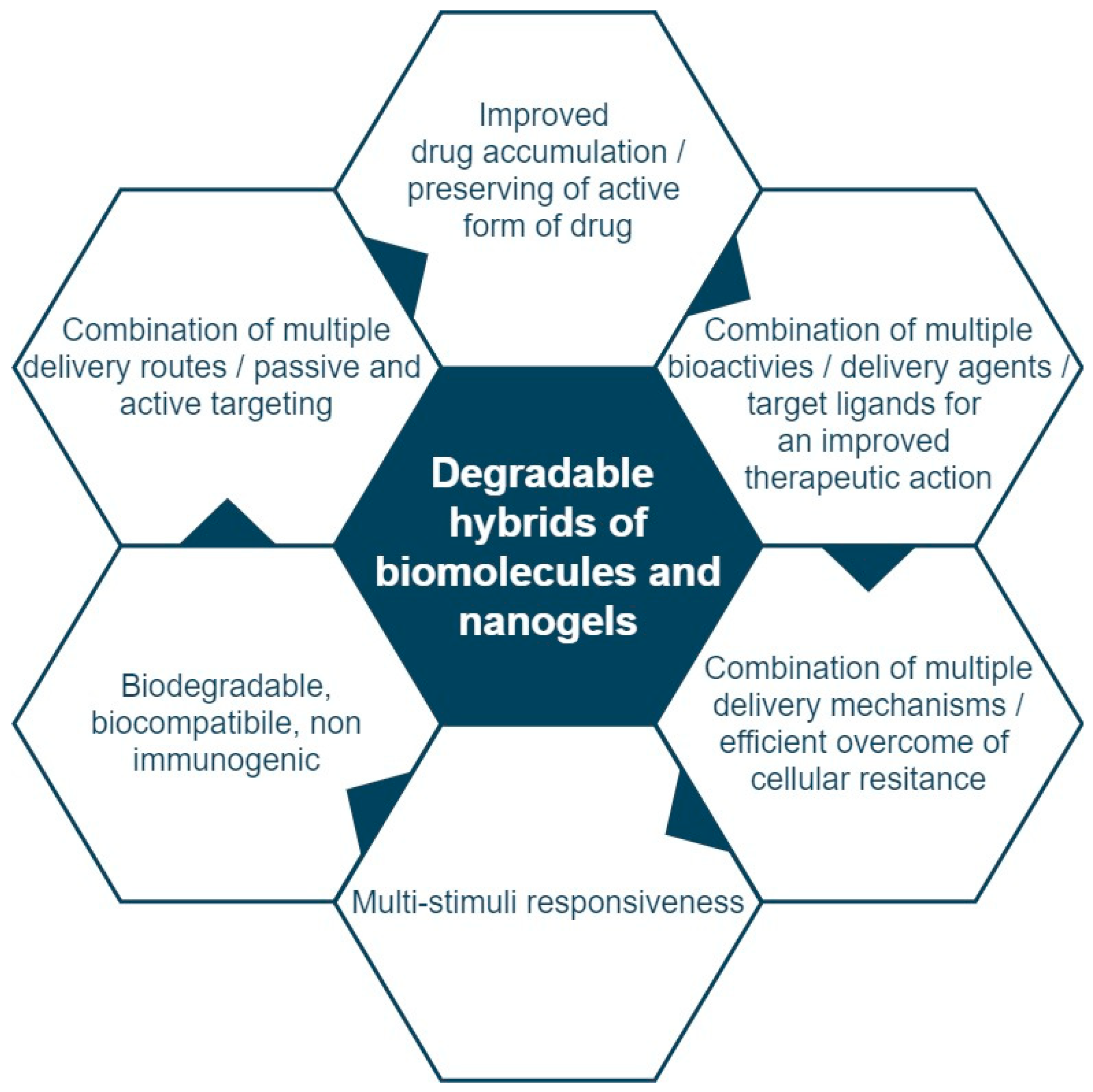



Molecules Free Full Text Recent Advances In Degradable Hybrids Of Biomolecules And Ngs For Targeted Delivery Html




Hydrophilic Vs Hydrophobic Substances Cell Membranes Youtube



Cell Membrane Wikipedia



0 件のコメント:
コメントを投稿